
3-Amino-4-hydroxybenzoic acid synthesis
- Product Name:3-Amino-4-hydroxybenzoic acid
- CAS Number:1571-72-8
- Molecular formula:C7H7NO3
- Molecular Weight:153.14
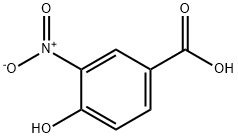
616-82-0

1571-72-8
The general procedure for the synthesis of 3-amino-4-hydroxybenzoic acid from 4-hydroxy-3-nitrobenzoic acid was as follows: tin(II) chloride (207 mg, 1.09 mmol), 12 N hydrochloric acid (1.5 mL), and 4-hydroxy-3-nitrobenzoic acid (100 mg, 0.55 mmol) were sequentially added to the reaction system at 0 °C. The reaction mixture was heated to reflux for 1 hour. After completion of the reaction, water was added to the mixture and the pH was adjusted to 1 with 2 N sodium hydroxide solution. the precipitate was collected by filtration and washed with distilled water. The filtrate was concentrated under vacuum and methanol was added to the residue. The precipitate formed was filtered again and the filtrate was concentrated under vacuum. Finally, the residue was purified by silica gel column chromatography using dichloromethane and methanol (5:1, v/v) as eluent to afford the target compound 3-amino-4-hydroxybenzoic acid (82 mg, 98% yield) as a brown solid. Its 1H NMR (500 MHz, DMSO-d6) data were as follows: δ 11.30 (brs, 1H), 9.42 (brs, 3H), 7.79 (s, 1H), 7.60 (d, 1H, J = 8.0 Hz), 7.07 (d, 1H, J = 8.0 Hz).

616-82-0
238 suppliers
$10.00/5g

1571-72-8
275 suppliers
$8.00/1g
Yield:1571-72-8 98%
Reaction Conditions:
with hydrogenchloride;tin(ll) chloride at 0; for 1 h;Reflux;
Steps:
3-Amino-4-hydroxybenzoic acid (1)
To tin(II) chloride (207 mg, 1.09 mmol), 12N-HCl (1.5 mL) and 4-hydroxy-3-nitrobenzoic acid (100 mg, 0.55 mmol) were added successively at 0°C, and the reaction mixture was refluxed for 1 h. After addition of water, the reaction mixture was adjusted to pH 1 using 2N NaOH. The precipitates obtained were filtered and washed with water. The filtrate was concentrated in vacuo, and methanol was added to the residue. The formed precipitate was filtered, and the filtrate was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography using methylene chloride and methanol (5:1) as eluent to obtain compound 1 (82 mg, 98%) as a brown solid [1H NMR (500 MHz, DMSO-d6) δ 11.30 (brs, 1 H), 9.42 (brs, 3 H), 7.79 (s, 1 H), 7.60 (d, 1 H, J = 8.0 Hz), 7.07 (d, 1 H, J = 8.0 Hz)].
References:
Kim, Min Jo;An, Hye Jin;Kim, Dae Hyun;Lee, Bonggi;Lee, Hye Jin;Ullah, Sultan;Kim, Su Jeong;Jeong, Hyoung Oh;Moon, Kyoung Mi;Lee, Eun Kyeong;Yang, Jungho;Akter, Jinia;Chun, Pusoon;Moon, Hyung Ryong;Chung, Hae Young [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 4,p. 684 - 688] Location in patent:supporting information

861550-32-5
3 suppliers
inquiry

1571-72-8
275 suppliers
$8.00/1g

91004-38-5
13 suppliers
$88.00/250 mg

1571-72-8
275 suppliers
$8.00/1g
94-26-8
601 suppliers
$10.00/5g

1571-72-8
275 suppliers
$8.00/1g

96-99-1
518 suppliers
$5.00/5g

1571-72-8
275 suppliers
$8.00/1g