
Methyl 3-amino-4-hydroxybenzoate synthesis
- Product Name:Methyl 3-amino-4-hydroxybenzoate
- CAS Number:536-25-4
- Molecular formula:C8H9NO3
- Molecular Weight:167.16

67-56-1

1571-72-8

536-25-4
1. 3-Amino-4-hydroxybenzoic acid (0.40 g, 2.61 mmol) was dissolved in anhydrous methanol (10 mL), and trimethylsilyl chloride (TMSCl, 0.75 mL, 5.94 mmol) was added. 2. The reaction mixture was stirred at 55°C for 48 h. 3. Upon completion of the reaction, the solvent was removed by evaporation under reduced pressure. 4. The crude product was purified by silica gel column chromatography. The crude product was purified by silica gel column chromatography using ethyl acetate as eluent to afford methyl 3-amino-4-hydroxybenzoate (0.25 g, 1.50 mmol, 57% yield) as a white solid. 5. Methyl 3-amino-4-hydroxybenzoate (0.20 g) was dissolved in anhydrous tetrahydrofuran (THF, 10 mL), and methyl 3-amino-4-hydroxybenzoate was added to methyl 3-amino-4-hydroxybenzoate, 0.26 g, 1.5 mL, and methyl 3-amino-4-hydroxybenzoate, 0.20 g, 2.5 mL, 2.4 mL, 2.5 mL. (0.26 g, 1.46 mmol). 6. The reaction mixture was stirred at room temperature overnight. 7. Upon completion of the reaction, the solvent was removed by evaporation under reduced pressure. 8. The residue was treated with water (15 mL) and extracted with ethyl acetate (3 × 15 mL). 9. The organic layers were combined, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. 10. The crude product was recrystallized from ethanol to give 2-thioalkylbenzo[d ]oxazole-5-carboxylic acid methyl ester (21, 0.22 g, 1.05 mmol, 88% yield) as a brown solid with a melting point of 203-205°C. The crude product was extracted with ethanol.

67-56-1
781 suppliers
$7.29/5ml-f

1571-72-8
275 suppliers
$8.00/1g

536-25-4
197 suppliers
$11.00/250mg
Yield:536-25-4 100%
Reaction Conditions:
with sulfuric acid for 24 h;Reflux;
Steps:
Step1: preparation of methyl 4-amino-3-nitrobenzoate (12).
General procedure: Toa solution of 4-amino-3-nitrobenzoic acid (11, 500 mg, 2.75 mmol) inMeOH (20 mL), a catalytic amount of concentrated sulfuric acid was added in a dropwisefashion. The mixture was heated at reflux temperature overnight. After beingcooled, the reaction was quenched by saturated NaHCO3solution andextracted by ethyl acetate.The organic layer was separated and dried over anhydroussodium sulfate. Filtration and concentrationin vacuogave 12 (519 mg, 97%); yellow solid
References:
Jaikhan, Pattaporn;Buranrat, Benjaporn;Itoh, Yukihiro;Chotitumnavee, Jiranan;Kurohara, Takashi;Suzuki, Takayoshi [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 10,p. 1173 - 1176] Location in patent:supporting information

99-42-3
189 suppliers
$8.00/5g

536-25-4
197 suppliers
$11.00/250mg

67-56-1
781 suppliers
$7.29/5ml-f
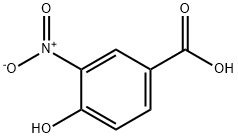
616-82-0
238 suppliers
$10.00/5g

536-25-4
197 suppliers
$11.00/250mg
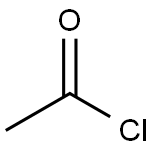
75-36-5
592 suppliers
$17.92/100G

1571-72-8
275 suppliers
$8.00/1g

536-25-4
197 suppliers
$11.00/250mg

18107-18-1
210 suppliers
$33.00/5g

1571-72-8
275 suppliers
$8.00/1g

536-25-4
197 suppliers
$11.00/250mg