
3-bromo-6-nitroquinoline synthesis
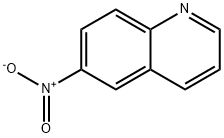
- Product Name:3-bromo-6-nitroquinoline
- CAS Number:7101-95-3
- Molecular formula:C9H5BrN2O2
- Molecular Weight:253
613-50-3

7101-95-3
120287-30-1
General procedure for the synthesis of 3-bromo-6-nitroquinoline and 8-bromo-6-nitroquinoline from 6-nitroquinoline: a mixed solution of 6-nitroquinoline (28.1 g, 161 mmol) and N-bromosuccinimide (28.7 g, 161 mmol) in acetic acid (280 mL) was heated and reacted for 17 hours at 50 °C. Upon completion of the reaction, the precipitated solid was collected by filtration and washed sequentially with ether, water and then ether to give 14.7 g (27% yield) of Intermediate 2 (93% purity). The organic phase was concentrated to dryness and the residue was purified by silica gel column chromatography using a mobile phase gradient (from 50% petroleum ether/50% dichloromethane to 100% dichloromethane). The pure grades were collected and the solvent concentrated to give 2.25 g (4% yield) of intermediate 2 and 16.6 g of residue. The residue was purified by a second silica gel column chromatography with a mobile phase of 50% petroleum ether/9:1:0.2 cyclohexane/ethyl ether/dichloromethane. The pure grades were collected and the solvent was concentrated to give 14.1 g (28% yield) of Intermediate 1.
Yield: 38.2%
Reaction Conditions:
with acetic acid at 110; for 2 h;Skraup Quinoline Synthesis;
Steps:
4.1.2.1.53. 3-Bromo-6-nitroquinoline (32)
4-nitroaniline (5.0 g,36.2 mmol) in acetic acid (35 mL) was treated with 2,2,3 tribromopropanal(11.7 g, 39.8 mmol) and the mixture was heated at110 °C for 2 h. AcOH was evaporated in vacuum followed byextraction of crude with ethyl acetate and water. Organic layer waswashed with sat. NaHCO3 and brine, respectively and dried overNa2SO4. Crude product was purified by column chromatographyusing ethyl acetate and hexane as eluting solvents; Yield =38.2%(3.5 g); 1H NMR (400 MHz, DMSO-d6) δ 9.23 (dt, J =17.1, 8.6 Hz, 1H),9.14 (d, J= 2.4 Hz, 1H), 8.89-8.73 (m, 2H), 7.86 (dd, J=8.3, 4.2 Hz,1H); HRMS Calculated for C9H6BrN2O2+ m/z 252.9607, found mass= 252.9611.
References:
Contreras, Jacob I.;Ezell, Edward L.;Garrison, Jered C.;Kizhake, Smitha;Mallareddy, Jayapal Reddy;Napoleon, John V.;Natarajan, Amarnath;Radhakrishnan, Prakash;Rajesh, Christabelle;Rana, Sandeep;Sagar, Satish;Singh, Sarbjit;Sonawane, Yogesh A. [European Journal of Medicinal Chemistry,2021,vol. 222]

613-50-3
237 suppliers
$10.00/5g

7101-95-3
59 suppliers
inquiry

696611-46-8
26 suppliers
$192.00/100mg

