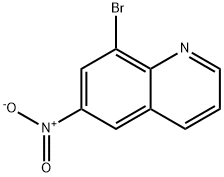
8-bromo-6-nitroquinoline synthesis
- Product Name:8-bromo-6-nitroquinoline
- CAS Number:120287-30-1
- Molecular formula:C9H5BrN2O2
- Molecular Weight:253.05
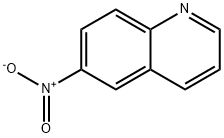
613-50-3

120287-30-1
Step 1. Preparation of 8-bromo-6-nitroquinoline To a reaction flask containing 6-nitroquinoline (4 g, 23 mmol) and concentrated sulfuric acid (20 ml), N-bromosuccinimide (5.31 g, 29.9 mmol) was added. The reaction mixture was heated to 60 °C in an oil bath with continuous stirring for 6 hours. After completion of the reaction, the mixture was cooled in a refrigerator overnight. The cooled crude reaction mixture was slowly poured into a beaker containing ice (250 ml). The pH of the mixture was adjusted to about 10 by batchwise addition of solid sodium bicarbonate followed by dropwise addition of saturated sodium bicarbonate solution.During this process, ethyl acetate (60 ml) was added to facilitate phase separation. The mixture was filtered to remove insoluble impurities and the filtrate was transferred to a partition funnel. Ethyl acetate (100 ml) was added to the partition funnel and shaken well to achieve separation of the two phases. The organic phase was separated and washed with an equal volume of saturated brine. The ethyl acetate phase was collected and the aqueous phase was back-extracted with ethyl acetate (2 x 100 ml). All organic phases were combined and concentrated under reduced pressure to give a solid product. The solid from the above filtration was dissolved in hot ethyl acetate (60 ml) and cooled to room temperature. The organic phase was dried by adding anhydrous magnesium sulfate and the desiccant was removed by filtration. The filtrate was concentrated under reduced pressure and the crude product obtained was combined with the product obtained from the aqueous phase post-treatment. Recrystallization by hot ethyl acetate/hexane mixed solvent gave the target product 8-bromo-6-nitroquinoline as a yellow powder (2.05 g). Mass spectral analysis showed (M + H)+ = 253/255 m/z.
Yield: 28% , 4%
Reaction Conditions:
with N-Bromosuccinimide;acetic acid at 50; for 17 h;
Steps:
A1.a Preparation of intermediates 1 and 2
A solution of 6-nitroquinoline (28.1 g; 161 mmol) and A/-bromosuccinimide (28.7 g; 161 mmol) in acetic acid (280 ml) was heated at 50°C for 17 hours. The precipitate solid was filtered and washed with Et20, water and then Et20 to afford 14.7g g (27%) of intermediate 2 (purity 93%) . The organic layer was evaporated to dryness and the residue was purified by chromatography over silica gel (mobile phase gradient from 50% petroleum ether, 50% DCM to 100% DCM). The pure fractions were collected and the solvent was evaporated, yielding 2.25 g (4 %) of intermediate 2 and 16.6g of a residue that was submitted to a second purification by chromatography over silica gel (mobile phase 50% petroleum9/1/0.2 cyclohexane/ diethyl ether/ DCM). The pure fractions were collected and the solvent was evaporated, yielding 14.1 g (28%) of intermediate 1.
References:
ASTEX THERAPEUTICS LIMITED;BERDINI, Valerio;ANGIBAUD, Patrick René;WOODHEAD, Steven John;SAXTY, Gordon WO2013/61074, 2013, A1 Location in patent:Page/Page column 147

613-50-3
237 suppliers
$10.00/5g

120287-30-1
37 suppliers
inquiry

88609-21-6
3 suppliers
inquiry

120287-30-1
37 suppliers
inquiry

91-22-5
410 suppliers
$10.00/25g

120287-30-1
37 suppliers
inquiry

88609-20-5
7 suppliers
inquiry

120287-30-1
37 suppliers
inquiry
