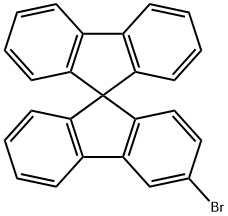
3-DroMo-9,9'-spirobifluorene synthesis
- Product Name:3-DroMo-9,9'-spirobifluorene
- CAS Number:1361227-58-8
- Molecular formula:C25H15Br
- Molecular Weight:395.29

2052-07-5
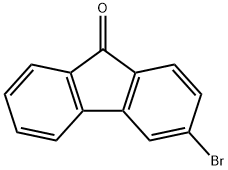
2041-19-2

1361227-58-8
The synthesis of the compound was carried out in two steps. Step 1: 2-Bromobiphenyl (1.05 eq., 4.0 g, 16.5 mmol) was dissolved in anhydrous ether (102 mL), and after the solution was cooled to -60 °C, n-butyllithium (n-BuLi, 1.16 eq.) was added slowly and dropwise. After maintaining this temperature for 10 minutes, a white precipitate was observed to be generated and the reaction mixture was subsequently warmed to room temperature to dissolve the precipitate. Next, 3-bromo-9H-fluoren-9-one was added and the reaction mixture was stirred at 45 °C overnight. After completion of the reaction, the reaction was quenched by adding 5% aqueous ammonium chloride solution (260 mL) and the organic phase was extracted with ether to give the alcohol intermediate (7.0 g). Step 2: The above alcohol intermediate was dissolved in acetic acid (141 mL) and hydrolyzed by adding 10% HCl/dioxane solution (78 mL, 10 mol%, 20 eq.). After evaporation to remove the solvent, the residual solid was purified by normal-phase fast chromatography to finally obtain 3-bromo-9,9'-spirobifluorene (5.86 g, 94% yield).

2052-07-5
387 suppliers
$16.30/1g

2041-19-2
110 suppliers
$16.00/250mg

1361227-58-8
101 suppliers
$18.00/100mg
Yield:1361227-58-8 94%
Reaction Conditions:
Stage #1:2-Bromobiphenyl with n-butyllithium in diethyl ether at -60;
Stage #2:3-bromofluoren-9-one in diethyl ether at 45;
Stage #3: with hydrogenchloride;acetic acid in 1,4-dioxane
Steps:
1.2 3-bromo-SBF
[0090] This compound was made in two steps from 3-bromofluorenone obtained in step 1. First, 2-bromobiphenyl (1.05 equivalents, 4.0 g, 16.5 mmol) is solubilised in 102 ml of anhydrous diethyl ether. This solution is cooled to -60°C and n-BuLi (1.16 eq.) is added dropwise. After 10 min at this temperature, a white precipitate appeared which was redissolved while the medium was warmed to room temperature. 3-Bromofluorenone was then added and the reaction mixture was kept at 45 °C for one night. [0091] After addition of NH4Cl (5% aq., 260 ml) and extraction with diethyl ether, 7.0 g of an alcohol was obtained. This solid was solubilised in 141 ml of acetic acid and hydrolyzed by the addition of 78 ml of HCl/dioxane (10 % mol., 20 eq.). After evaporation of the solvents, the solid was subjected to normal phase flash chromatography to afford 5.86 g of the target compound (94 % yield).
References:
SOLVAY SA;The designation of the inventor has not yet been filed EP2574608, 2013, A1 Location in patent:Paragraph 0088; 0089; 0090; 0091

2041-19-2
110 suppliers
$16.00/250mg
![Lithium, [1,1'-biphenyl]-2-yl-](/CAS/20200119/GIF/55365-18-9.gif)
55365-18-9
1 suppliers
inquiry

1361227-58-8
101 suppliers
$18.00/100mg

2041-19-2
110 suppliers
$16.00/250mg

82214-69-5
25 suppliers
$255.00/50mL

1361227-58-8
101 suppliers
$18.00/100mg

2041-19-2
110 suppliers
$16.00/250mg
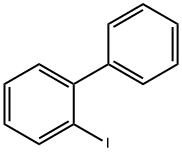
2113-51-1
265 suppliers
$6.00/1g

1361227-58-8
101 suppliers
$18.00/100mg

135776-98-6
73 suppliers
inquiry

1361227-58-8
101 suppliers
$18.00/100mg