
(2,7-BIS(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-9,9-DIOCTYLFLUORENE) synthesis
- Product Name:(2,7-BIS(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-9,9-DIOCTYLFLUORENE)
- CAS Number:196207-58-6
- Molecular formula:C41H64B2O4
- Molecular Weight:642.57
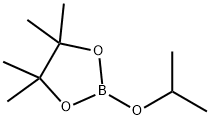
61676-62-8

198964-46-4

196207-58-6
Under argon protection, 2,7-dibromo-9,9-dioctylfluorene (21.9 g, 40 mmol) and 250 mL of anhydrous tetrahydrofuran were added to a 500 mL three-neck flask. The reaction solution was cooled to -78°C (using a liquid nitrogen bath) and a n-hexane solution of n-butyllithium (48 mL, 2.5 M, 120 mmol) was added slowly and dropwise. After stirring at -78°C for 2 h, 2-isopropyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (26 g, 140 mmol) was added all at once and the reaction mixture was allowed to naturally rise to room temperature. The reaction was continued for 20 hours. After completion of the reaction, the mixture was poured into water and extracted with dichloromethane. The organic phase was washed five times with saturated saline, the organic phase was separated, dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by column chromatography using silica gel as stationary phase and petroleum ether/dichloromethane as mobile phase. After purification, 43.8 g of white solid product was obtained in 80% yield.

61676-62-8
322 suppliers
$10.00/5g

198964-46-4
187 suppliers
$16.00/5g

196207-58-6
161 suppliers
$37.50/250 mg
Yield:196207-58-6 80%
Reaction Conditions:
Stage #1:2,7-dibromo-9,9-dioctylfluorene with n-butyllithium in tetrahydrofuran;hexane at -78; for 2 h;Inert atmosphere;
Stage #2:2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;hexane at 20; for 20 h;Inert atmosphere;
Steps:
3 Example 3
Preparation of 2,7-bis (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -9,9-dioctylfluorene (M-2)
Under argon protection,In a 500 mL long three-necked flask,Was added 2,7-dibromo-9,9-dioctylfluorene (21.9 g, 40 mmol)And 250 mL of anhydrous tetrahydrofuran,The reaction solution was cooled to -78 ° C with liquid nitrogen,A n-hexane solution of n-butyllithium (48 mL, 2.5 M, 120 mmol) was slowly added dropwise,After stirring at -78 ° C for 2 hours,2-isopropyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (26 g, 140 mmol) was injected in one portion,Let it naturally rise to room temperature,Continue to react for 20 h.The reaction mixture was poured into water and extracted with methylene chloride. The mixture was washed five times. The organic phase was separated, dried, filtered and the solvent was dried. The residue was purified by column chromatography. The crude product was purified by silica gel as a stationary phase and petroleum ether / dichloromethane as the mobile phase. After purification, 43.8 g of a white solid was obtained in 80% yield.
References:
South China University of Technology;Guo Ting;Fu Denghao;Ying Lei;Yang Wei;Peng Junbiao;Cao Yong CN106588869, 2017, A Location in patent:Paragraph 0053; 0054; 0055; 0056
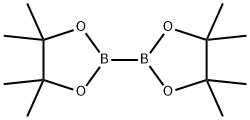
73183-34-3
583 suppliers
$6.00/5g

198964-46-4
187 suppliers
$16.00/5g

196207-58-6
161 suppliers
$37.50/250 mg

61676-62-8
322 suppliers
$10.00/5g

1059179-65-5
11 suppliers
inquiry

196207-58-6
161 suppliers
$37.50/250 mg

111-83-1
499 suppliers
$12.00/25g

196207-58-6
161 suppliers
$37.50/250 mg

16433-88-8
369 suppliers
$6.00/5g

196207-58-6
161 suppliers
$37.50/250 mg