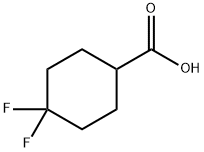
4,4-Difluorocyclohexanecarboxylic acid synthesis
- Product Name:4,4-Difluorocyclohexanecarboxylic acid
- CAS Number:122665-97-8
- Molecular formula:C7H10F2O2
- Molecular Weight:164.15

178312-47-5

122665-97-8
The general procedure for the synthesis of 4,4-difluorocyclohexanecarboxylic acid from ethyl 4,4-difluorocyclohexanecarboxylate was as follows: ethyl 4,4-difluorocyclohexanecarboxylate (0.385 g, 2.003 mmol) was dissolved in THF (12 mL), and H2O (6 mL) was added, followed by lithium hydroxide monohydrate (0.420 g, 10.02 mmol). The reaction mixture was stirred vigorously at room temperature overnight. Upon completion of the reaction, the mixture was diluted with EtOAc and the pH was adjusted to 4 with 1 M HCl. The organic and aqueous layers were separated, and the aqueous layer was then extracted with EtOAc (1 time). The organic phases were combined, washed with brine, dried over MgSO4 and concentrated to dryness to give 4,4-difluorocyclohexanecarboxylic acid (318 mg, 97% yield) as a white solid.1H NMR (400 MHz, DMSO-d6): δ 12.28 (s, 1H), 2.40 (m, 1H), 2.02-1.75 (m, 6H), 1.59 (m, 2H) .

178312-47-5
183 suppliers
$8.00/1g

122665-97-8
293 suppliers
$18.00/250mg
Yield:122665-97-8 97%
Reaction Conditions:
with water;lithium hydroxide in tetrahydrofuran at 20;
Steps:
12 Example 12
A solution of ethyl 4,4-difluorocyclohexanecarboxylate (0.385 g, 2.003 mmol) in THF (12 mL) was treated with H2O (6 mL) followed by lithium hydroxide monohydrate (0.420 g, 10.02 mmol) and the mixture stirred vigorously at RT overnight.
The mixture was treated with EtOAc, acidified with 1M HCl until pH 4, the layers separated and the aqueous layer extracted with additional EtOAc (1*).
The combined organics were washed with brine, dried over MgSO4 and concentrated to dryness to afford 4,4-difluorocyclohexanecarboxylic acid (318 mg, 97%) as a white solid. 1H NMR (400 MHz, DMSO-d6): δ 12.28 (s, 1H), 2.40 (m, 1H), 2.02-1.75 (m, 6H), 1.59 (m, 2H).
References:
Deciphera Pharmaceuticals, LLC;Flynn, Daniel L.;Caldwell, Timothy Malcolm;Kaufman, Michael D.;Patt, William C.;Samarakoon, Thiwanka;Vogeti, Lakshminarayana;Yates, Karen M. US2014/275080, 2014, A1 Location in patent:Paragraph 0428

17159-79-4
347 suppliers
$5.00/1g

122665-97-8
293 suppliers
$18.00/250mg

17159-79-4
347 suppliers
$5.00/1g

122665-97-8
293 suppliers
$18.00/250mg
350-44-7
1 suppliers
inquiry