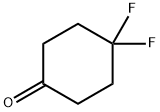
4,4-DIFLUOROCYCLOHEXANONE synthesis
- Product Name:4,4-DIFLUOROCYCLOHEXANONE
- CAS Number:22515-18-0
- Molecular formula:C6H8F2O
- Molecular Weight:134.12
![8,8-difluoro-1,4-dioxaspiro[4.5]decane](/CAS/20150408/GIF/176251-49-3.gif)
176251-49-3

22515-18-0
4.3. Synthesis of 4,4-difluorocyclohexanone (8): the acetonide 7 (20.0 g, 112.2 mmol) was suspended in 20% HCl aqueous solution (16 mL) and stirred vigorously for 3 hours at 100 °C. After the reaction was completed, it was cooled to room temperature and NaCl (~3 g) was added to the aqueous phase to dissolve it. Subsequently, the aqueous phase was extracted with CH2Cl2 (3 x 50 mL). The organic phases were combined, washed with 50 mL of water, dried over Na2SO4, and the solvent was slowly evaporated under reduced pressure at 20 °C. Due to the extreme instability of the product, the material initially obtained was a 1:1 mixture of product 8 and raw material 5. After repeating the above operation, the ratio of 8 to 5 in the mixture was increased to 7:3. After a number of repetitions, the final product 8 was obtained with 95% purity as white crystals (11.0 g, 71% yield) with a melting point of 31-32°C. The product was then dried over 50 mL of water and evaporated slowly at 20°C under reduced pressure.
![8,8-difluoro-1,4-dioxaspiro[4.5]decane](/CAS/20150408/GIF/176251-49-3.gif)
176251-49-3
55 suppliers
$8.00/250mg

22515-18-0
247 suppliers
$5.00/100mg
Yield:22515-18-0 93%
Reaction Conditions:
with hydrogenchloride;water at 100; for 12 h;
Steps:
84.2 Step 2. 4, 4-Difluorocyclohexanone (84c)
Compound 84b (1 g, 5.6 mmol) was dissolved in aq. HCl (2 mL, 2N) . The resulting mixture was stirred at 100 for 12 hrs. After cooling down to RT, the mixture was diluted with DCM (10 mL) and adjusted to pH = 78 with sat. NaHCO 3. The separated organic layer was washed with sat. NaHCO 3, dried over Na 2SO 4 and filtered. The filtrate was concentrated to dryness to afford the desired product as colorless oil (700 mg, 93%yield) . 1H NMR (400 MHz, CDCl 3) δ 2.56 -2.53 (m, 4H) , 2.36 -2.27 (m, 4H) . 19F NMR (376 MHz, CDCl 3) δ -100.27.
References:
LYNK PHARMACEUTICALS CO. LTD.;WAN, Zhaokui;VAZQUEZ, Michael Lawrence;LI, Xiaodong WO2020/119819, 2020, A1 Location in patent:Paragraph 00395
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
492 suppliers
$5.00/1g

22515-18-0
247 suppliers
$5.00/100mg

6317-49-3
101 suppliers
$18.00/10g

22515-18-0
247 suppliers
$5.00/100mg

539-47-9
270 suppliers
$10.00/1g

22515-18-0
247 suppliers
$5.00/100mg
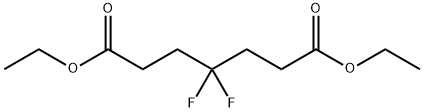
22515-16-8
93 suppliers
$13.00/250mg

22515-18-0
247 suppliers
$5.00/100mg