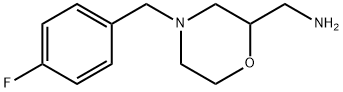
4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine synthesis
- Product Name:4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine
- CAS Number:112914-13-3
- Molecular formula:C12H17FN2O
- Molecular Weight:224.27

5455-98-1

22116-33-2
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
General procedure for the synthesis of 2-aminomethyl-4-(4-fluorobenzyl)-morpholine from N-(2,3-epoxypropyl)phthalimide and 2-(4-fluorobenzylamino)ethanol: 2-(4-fluorobenzylamino)ethanol (28.7 g, 0.17 mol) and N-(2,3-epoxypropyl)phthalimide (35.0 g, 0.13 mol) were added to the reaction kettle. The reaction was heated to 70-80°C and held for 3 hours. Subsequently, sulfuric acid was slowly added dropwise at 70-90°C and the reaction was continued at 125-145°C for 2.5 hours. Upon completion of the reaction, the mixture was transferred to cold water, cooled to 0°C, crystallized and filtered. The filtrate was neutralized with sodium hydroxide, extracted with chloroform, dried the organic phase and concentrated to dryness to give 4-(4-fluorobenzyl)-2-aminomethylmorpholine as a light yellow oil (yield: 75.1%, purity: 80.2%).
![1H-Isoindole-1,3(2H)-dione, 2-[[4-[(4-fluorophenyl)methyl]-2-morpholinyl]methyl]-](/CAS/20210305/GIF/636582-78-0.gif)
636582-78-0
0 suppliers
inquiry
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
158 suppliers
$5.00/250mg
Yield:112914-13-3 90%
Reaction Conditions:
with hydrazine in ethanol; for 3 h;Heating / reflux;
Steps:
7.D Example 7; Synthesis of 2-aminomethyl-4- (4-fluoro-benzyl) morphline (VII); D.)
16.2 g (265 [MMOL)] of 2-amino-ethanol, 27.3 g (220 [MMOL)] of [4-FLUORO-BENZALDEHYDE] and 27.6 g of sodium-hydrogen-carbonate are refluxed in 260 ml of methanol with stirring for 4 hours and then 5.00 g (132 [MMOL)] of [SODIUM-BOROHYDRIDE] is added at 0-5 [C] for 2 hours. The reaction mixture is allowed to warm to room temperature, after the evolution of hydrogen is stopped filtered and evaporated under reduced pressure. The residue is solved in [DICHLOROMETHANE,] washed 2 x with saturated sodium chloride solution, dried over sodium sulphate, filtered and evaporated under reduced pressure to give 32.8 g (88%) of [2- (4-] fluoro-benzyl-amino)-ethanol as colourless, viscous oil. B) To 15.0 g (88.7 [MMOL)] of [2- (4-FLUORO-BENZYL-AMINO)-ETHANOL] obtained in the above (A) step 8.65 g (92.5 [MMOL)] of [EPICHLOROHYDRIN] is added, the mixture stirred 4.5 hours, 66 g of concentrated sulphuric acid added during 15 minutes and warmed at 150 [C] for 30 minutes. After cooling the sulphuric acid solution is poured into ice/water, treated with saturated sodium hydroxide solution and extracted with chloroform. The extract is washed with saturated sodium chloride solution, dried over sodium sulphate, filtered and evaporated under reduced pressure, to give 16.1 g (75%) of [2-CHLORO-METHYL-4- (4-FLUORO-BENZYL)-] morpholine as yellow, viscous oil. C) 12.3 g (50.5 [MMOL)] of [2-CHLORO-METHYL-4- (4-FLUORO-BENZYL)-MORPHOLINE] obtained in the above (B) step is solved in 195 ml of [ANHYDROUS N, N-DIMETHYL-FORMAMIDE] and 10.6 g (57.2 [MMOL)] of potassium-phthalimide added to the solution. The suspension is [REFLUXED] for 1.5 hours, then cooled and filtered. The solvent is evaporated under reduced pressure, the residue solved in chloroform and washed with water four times, dried over sodium sulphate, filtered and evaporated under reduced pressure. The residue is recrystallized from isopropanol to give 13.5 g (75%) of nearly white [2- [4- (4-FLUORO-BENZYL)-MORPHOLINE-2YL-METHYL]-ISOINDOLE-] 1.3-dion crystals. Mp: 150-151 [C.] D) 10.0 g (28.1 [MMOL)] of [2- [4- (4-FLUORO-BENZYL)-MORPHOLINE-2YL-METHYL]-ISOINDOLE-1. 3-DION] obtained in the above (C) step is suspended in the solution of 5.32 g (106 [MMOL)] of [HYDRAZINE-MONOHYDRATE] and 170 mi of ethanol and refluxed for 3 hours. The precipitation is removed by filtration and the ethanol solvent is evaporated under reduced pressure. The residue is solved in water and the solution extracted with [DICHLOROMETHANE.] The organic phase is washed 2 x with water, dried over sodium sulphate, filtered and evaporated under reduced pressure, to give 5.67 g (90%) of the title compound as yellow transparent oil.
References:
WO2003/106440,2003,A2 Location in patent:Page 10-11

5455-98-1
177 suppliers
$25.00/5g

22116-33-2
101 suppliers
$35.00/200μmol
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
158 suppliers
$5.00/250mg

112913-94-7
82 suppliers
$8.00/10g
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
158 suppliers
$5.00/250mg
![Acetamide, N-[[4-(phenylmethyl)-2-morpholinyl]methyl]-](/CAS/20210305/GIF/112913-96-9.gif)
112913-96-9
2 suppliers
inquiry
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
158 suppliers
$5.00/250mg
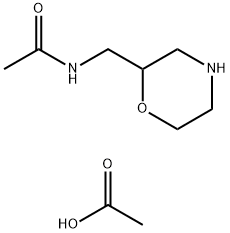
131322-23-1
0 suppliers
inquiry
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
158 suppliers
$5.00/250mg