4-Amino-2-chlorobenzoic acid synthesis
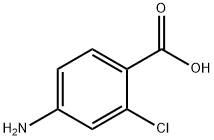
- Product Name:4-Amino-2-chlorobenzoic acid
- CAS Number:2457-76-3
- Molecular formula:C7H6ClNO2
- Molecular Weight:171.58

99-60-5

2457-76-3
The general procedure for the synthesis of 4-amino-2-chlorobenzoic acid from 2-chloro-4-nitrobenzoic acid was as follows: firstly, NaBH4 (1 mmol) was added as a reducing agent to methanol at room temperature and the reaction was carried out for 2 h. Ag (+1) was reduced to Ag (0). Subsequently, the Ag/MMT catalyst was obtained by filtration separation. In a standard reaction procedure, 2-chloro-4-nitrobenzoic acid (0.1 mmol), KOH (0.15 mmol), isopropanol (3 mL) and 1.01 wt% of Ag/MMT catalyst (50 mg) were mixed and the reaction was stirred at room temperature until completion (the reaction progress was monitored by GC). Upon completion of the reaction, the catalyst was removed by filtration. The product was extracted with ethyl acetate and washed repeatedly (3-4 times) with water to remove residual KOH. finally, the solvent in the extract was evaporated to dryness under vacuum to afford the target product 4-amino-2-chlorobenzoic acid.

99-60-5
330 suppliers
$19.10/100g

2457-76-3
301 suppliers
$5.00/1g
Yield:2457-76-3 88%
Reaction Conditions:
with isopropyl alcohol;potassium hydroxide at 20; for 2.5 h;Catalytic behavior;
Steps:
General procedure for the reduction in nitroarenes
First, NaBH4 (1 mmol) was used as a reducing agent forthe conversion of Ag (+ 1) to Ag (0) in methanol at roomtemperature for 2 h. After that, the Ag/MMT was filtered andused as a catalyst. Then, in a typical procedure, a mixtureof 4-nitrophenol (0.1 mmol), KOH (0.15 mmol), isopropylalcohol (3 mL), and Ag/MMT catalyst (50 mg) 1.01 wt%was stirred at room temperature for an appropriate time(Scheme 2). After the completion of the reaction (monitoredby GC), the catalyst was separated by filtration. The ensuingproduct was extracted with ethyl acetate and repeatedlywashed with water (3-4 times) to remove KOH. The resultingsolvent was evaporated to dryness in vacuum.
References:
Karami, Kazem;Rahimi, Mahzad;Dinari, Mohammad [Journal of the Iranian Chemical Society,2018,vol. 15,# 2,p. 281 - 291]

121-86-8
218 suppliers
$14.00/5g

2457-76-3
301 suppliers
$5.00/1g

38667-55-9
13 suppliers
$45.00/50mg

2457-76-3
301 suppliers
$5.00/1g

588-07-8
107 suppliers
$22.19/25g

2457-76-3
301 suppliers
$5.00/1g

5568-33-2
51 suppliers
inquiry

2457-76-3
301 suppliers
$5.00/1g