
N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide synthesis
- Product Name:N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide
- CAS Number:90357-51-0
- Molecular formula:C12H9F3N2O2
- Molecular Weight:270.21
![N-[4-Cyano-3-(trifluoromethyl)phenyl]-2-methacrylamide](/CAS/GIF/90357-53-2.gif)
90357-53-2
![N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide](/CAS/GIF/90357-51-0.gif)
90357-51-0
General procedure for the synthesis of N-[4-cyano-3-(trifluoromethyl)phenyl]methoxyacrylamide from N-(4-cyano-3-(trifluoromethyl)phenyl)methoxyacrylamide: N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methacrylamide (50.8 g, 0.2 mol) and phthalic anhydride (47.3 g, 0.32 mol) were dissolved in 350 ml of 1,2-dichloroethane. 50% hydrogen peroxide (23.1 g, 0.34 mol) was added slowly and dropwise at 35?40 °C. The progress of the reaction was monitored by HPLC until the content of the feedstock N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methylacrylamide decreased to less than 0.5% and the reaction was completed. Subsequently, 1,2-dichloroethane was removed by distillation to give the crude product. To the crude product, 300 g of water was added and neutralized by adding 26 g of sodium carbonate. Next, 13 g of sodium sulfate was added and filtered after stirring for 0.5 hours. The filter cake was washed with 50 g of water and dried to give the final product N-[4-cyano-3-(trifluoromethyl)phenyl]-2,3-epoxy-2-methylpropanamide 49 g in 90.7% yield.
![N-[4-Cyano-3-(trifluoromethyl)phenyl]-2-methacrylamide](/CAS/GIF/90357-53-2.gif)
90357-53-2
153 suppliers
$60.00/5 g
![N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide](/CAS/GIF/90357-51-0.gif)
90357-51-0
219 suppliers
$28.00/5G
Yield:90357-51-0 82%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in dichloromethane for 10 h;Heating / reflux;
Steps:
2 EXAMPLE 2 .N-[4-cyano-3-(trifluoromethyl)phenyl]-2-methyl-2-oxiranecarboxamide (5A)
460 mg of the amide (7) from the Example 1 was dissolved in 30 ml of dichloromethane. 560 mg of m-chloroperbenzoic acid (purity 70-75%) was added. The reaction mixture was stirred overnight at reflux. The reaction was monitored with HPLC. The reaction mixture was washed successively with 2×20 ml of a sodium sulfite solution, 2×20 ml of saturated aqueous NaHCO3 and 20 ml of brine. The organic layer was dried (Na2SO4), filtrated and evaporated under reduced pressure yielding the epoxide (5A) as yellow solid material. Isolated yield: 400 mg (82%). 1H- and 13C-NMR confirmed the expected structure. HPLC: 96% purity
References:
Thijs, Lambertus;Keltjens, Rolf;Ettema, Gerrit J. B. US2004/68135, 2004, A1 Location in patent:Page 12
![N-[4-Cyano-3-(trifluoromethyl)phenyl]-2-methacrylamide](/CAS/GIF/90357-53-2.gif)
90357-53-2
153 suppliers
$60.00/5 g
![N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide](/CAS/GIF/90357-51-0.gif)
90357-51-0
219 suppliers
$28.00/5G

38649-35-3
16 suppliers
inquiry
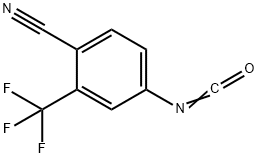
143782-18-7
31 suppliers
inquiry
![3-chloro-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide](/CAS/20200401/GIF/627484-44-0.gif)
627484-44-0
1 suppliers
inquiry
![N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide](/CAS/GIF/90357-51-0.gif)
90357-51-0
219 suppliers
$28.00/5G
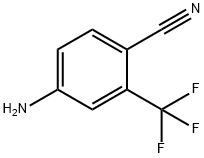
654-70-6
487 suppliers
$5.00/1g
![N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide](/CAS/GIF/90357-51-0.gif)
90357-51-0
219 suppliers
$28.00/5G