
Olmesartan Medoxomil synthesis
- Product Name:Olmesartan Medoxomil
- CAS Number:144689-63-4
- Molecular formula:C29H30N6O6
- Molecular Weight:558.59

144690-92-6
281 suppliers
$9.00/1g

144689-63-4
737 suppliers
$6.00/5mg
Yield:144689-63-4 100%
Reaction Conditions:
with acetic acid in water at 25; for 1 h;
Steps:
1.6 Example 1-6
Example 1-6 (0333) (0334) A mixture of TOLM (0.6 g, 0.75 mmol), sulfuric acid (0.08 g, 0.82 mmol) and 1:1 water-containing acetic acid (2.6 mL, 4.3 vol) was stirred at 25° C. for 1 hr. The reaction mixture was filtered, and the obtained solid was washed with 1:1 water-containing acetic acid (6.0 mL, 10 vol). The filtrates were combined and adjusted to pH 4-5 by adding 25% aqueous sodium carbonate solution. The mixture was partitioned by adding methylene chloride (6.0 mL, 10 vol). The aqueous layer was extracted with methylene chloride (3×5 mL). The organic layer was washed with water (2×5 mL) and saturated brine (5 mL), and concentrated under reduced pressure. The concentrated residue was purified by silica gel column chromatography (5-6% methanol/methylene chloride) and recrystallized from acetonitrile to give OLM (0.45 g, yield 100%). (0335) melting point: 174.5° C.-175.2° C.; (0336) IR (KBr): νmax=2969, 1831, 1706, 1475, 1226, 1134, 760 cm-1; (0337) 1H NMR (DMSO-d6): δ=7.70-7.63 (m, 2H), 7.59-7.52 (m, 2H). (0338) 7.04 (d, J=8 Hz, 2H), 6.85 (d, J=8.4 Hz, 2H), 5.42 (s, 2H), 5.21 (s, 1H), 5.05 (s, 2H), 2.60 (t, J=7.6 Hz, 2H), 2.07 (s, 3H), 1.60-1.55 (m, 2H), 1.47 (s, 6H), 0.87 (t, J=7.2 Hz, 3H); (0339) Mass: 559 [M+H]+.
References:
US2015/239854,2015,A1 Location in patent:Page/Page column 40-41
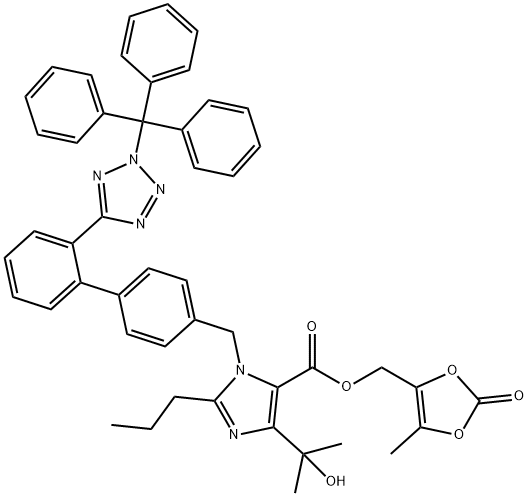
1020157-01-0
69 suppliers
$145.00/10mg

144689-63-4
737 suppliers
$6.00/5mg

80841-78-7
544 suppliers
$10.00/250mg
172875-59-1
51 suppliers
inquiry

144689-63-4
737 suppliers
$6.00/5mg

144690-92-6
281 suppliers
$9.00/1g

76-84-6
444 suppliers
$6.00/25g

144689-63-4
737 suppliers
$6.00/5mg
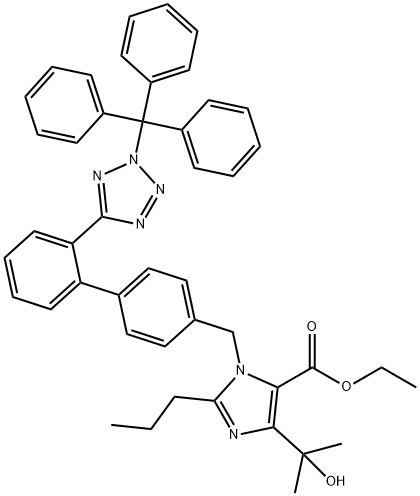
![1H-IMidazole-5-carboxylic acid, 4-(1-hydroxy-1-Methylethyl)-2-propyl-1-[[2'-[1-(triphenylMethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]Methyl]-, ethyl ester](/CAS/GIF/144690-33-5.gif)
144690-33-5
141 suppliers
inquiry

144689-63-4
737 suppliers
$6.00/5mg