| Identification | More | [Name]
3-FLUORO-2-FORMYLPYRIDINE | [CAS]
31224-43-8 | [Synonyms]
3-FLUORO-2-FORMYLPYRIDINE
3-FLUOROPYRIDINE-2-CARBALDEHYDE
3-FLUOROPYRIDINE-2-CARBOXALDEHYDE
3-Fluoropyridine-2-carboxaldehyde 97%
3-Fluoropyridine-2-carboxaldehyde97%
2-Pyridinecarboxaldehyde,3-fluoro-(9CI)
3-FLURO-2-FORMYLPYRIDINE
3-FLUOROPYRIDINE-2-CARBOXALDEHYDE/3-FLUORO-2-FORMYLPYRIDINE
3-FLUORO-2-FORMYLPYRIDINE ,98%
3-Fluoro-2-pyridinecarboxaldehyde | [EINECS(EC#)]
690-026-4 | [Molecular Formula]
C6H4FNO | [MDL Number]
MFCD07781234 | [Molecular Weight]
125.1 | [MOL File]
31224-43-8.mol |
| Chemical Properties | Back Directory | [Melting point ]
49 °C | [Boiling point ]
112°C/50mmHg(lit.) | [density ]
1.269±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [solubility ]
soluble in Methanol | [form ]
Solid | [pka]
1.25±0.10(Predicted) | [color ]
White to Green to Brown | [Water Solubility ]
Slightly soluble in water. | [InChI]
InChI=1S/C6H4FNO/c7-5-2-1-3-8-6(5)4-9/h1-4H | [InChIKey]
OZIMPUNGBUYCSP-UHFFFAOYSA-N | [SMILES]
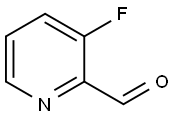
C1(C=O)=NC=CC=C1F | [CAS DataBase Reference]
31224-43-8(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Uses]
3-Fluoropyridine-2-carboxaldehyde is used to prepare taxoids derived from 9β-dihydrobaccatin-9,10-acetals with anti-tumor activities. It is also an intermediate used to synthesize S-3578 derivatives with potent activities against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa. | [Synthesis Reference(s)]
Tetrahedron, 39, p. 2009, 1983 DOI: 10.1016/S0040-4020(01)91919-2 | [Synthesis]
To a solution of DABCO (8.8 g, 78.2 mmol) in anhydrous ether (250 mL) was slowly added n-BuLi (1.6 M hexane solution, 49 mL, 78.2 mmol) at -25 °C. The reaction mixture was stirred between -25 and -10°C for 45 minutes and subsequently cooled to -70°C. At -70 °C, 3-fluoropyridine (5.9 mL, 71 mmol) was added dropwise to the above reaction solution. The reaction temperature was maintained between -70 and -60 °C and stirring was continued for 1.5 hours. After that, DMF (11.0 mL, 2.0 eq.) was added to the reaction system. After stirring at -70 °C for 1.0 h, the reaction was quenched by slow addition of water (150 mL) and gradually warmed to room temperature. Upon completion of the reaction, the organic and aqueous layers were separated and the aqueous layer was extracted with dichloromethane (5 x 100 mL). All organic layers were combined, washed with saturated saline and dried with anhydrous sodium sulfate. After the solvent was removed by concentration under reduced pressure, the crude product was purified by silica gel column chromatography using ethyl acetate/hexane gradient elution to afford 3-fluoropyridine-2-aldehyde (5.4 g, 55-60% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.54-7.57 (m, 2H), 8.61 (d, J = 2.20 Hz, 1H), 10.20 (s, 1H). | [References]
[1] Patent: US2010/227894, 2010, A1. Location in patent: Page/Page column 55
[2] Patent: WO2006/114706, 2006, A1. Location in patent: Page/Page column 47-48
[3] Tetrahedron, 1983, vol. 39, # 12, p. 2009 - 2021
[4] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 23, p. 6998 - 7003
[5] Bioorganic and Medicinal Chemistry Letters, 2011, vol. 21, # 6, p. 1852 - 1856 |
|
|