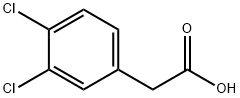
| Identification | More | [Name]
Methyl 3,4-dichlorophenylacetate | [CAS]
6725-44-6 | [Synonyms]
3,4-DICHLOROPHENYLACETIC ACID METHYL ESTER
METHYL 3,4-DICHLOROPHENYLACETATE
Dichlorophenyl acetic acid methyl ester
Methyl 3,4-dichlorophenylacetate, 97+%
Methyl2-(3,4-dichlorophenyl)acetate | [Molecular Formula]
C9H8Cl2O2 | [MDL Number]
MFCD00800680 | [Molecular Weight]
219.06 | [MOL File]
6725-44-6.mol |
| Chemical Properties | Back Directory | [Appearance]
clear colorless liquid | [Melting point ]
25-27 | [Boiling point ]
314.25°C (rough estimate) | [density ]
1.54-1.542 | [refractive index ]
1.54-1.542
| [storage temp. ]
Sealed in dry,Room Temperature | [form ]
Liquid After Melting | [color ]
Clear colorless to light yellow | [CAS DataBase Reference]
6725-44-6(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [Hazard Note ]
Irritant | [HS Code ]
29163990 |
| Hazard Information | Back Directory | [Chemical Properties]
clear colorless liquid | [Synthesis]
The general procedure for synthesizing methyl 3,4-dichlorophenylacetate from methanol and 3,4-dichlorophenylacetic acid was as follows: referring to Synthesis Example 1, methanol (59 mL, 3.0 equiv) was added to a solution of 1,2-dichloroethane (400 mL) containing 3,4-dichlorophenylacetic acid (100 g, 0.488 mol). The mixed solution was heated to 50 °C, followed by the slow dropwise addition of concentrated sulfuric acid (10 mL) over 15 min. After the dropwise addition was completed, the reaction temperature was maintained at 50°C and stirring was continued for 1.5 hours. Upon completion of the reaction, the reaction solution was cooled to room temperature and a partitioning operation was performed to separate and remove the sulfuric acid layer. The resulting organic layer was washed sequentially with water, saturated aqueous sodium bicarbonate solution and saturated brine, followed by drying with anhydrous magnesium sulfate. After drying, the desiccant was removed by filtration and the solvent was removed by distillation, resulting in the target product methyl 3,4-dichlorophenylacetate (105 g, yield: 98%) in the form of colorless oil. The structure of the product was confirmed by 1H-NMR (300 MHz, CDCl3) with chemical shifts δ: 3.59 (s, 2H), 3.71 (s, 3H), 7.12 (dd, J = 8.4 Hz, 1.8 Hz, 1H), 7.38-7.41 (m, 2H). | [References]
[1] Bioorganic and Medicinal Chemistry, 2003, vol. 11, # 13, p. 2843 - 2866
[2] Journal of Medicinal Chemistry, 2001, vol. 44, # 17, p. 2701 - 2706
[3] Journal of Medicinal Chemistry, 2010, vol. 53, # 9, p. 3618 - 3625
[4] Patent: US2003/225283, 2003, A1. Location in patent: Page 12-13
[5] Patent: EP2246343, 2010, A1. Location in patent: Page/Page column 10 |
|
|